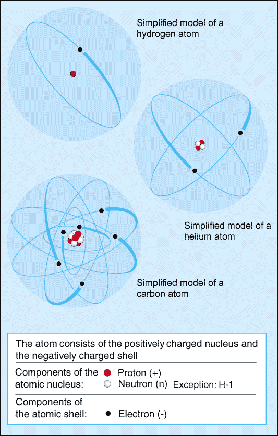
Smallest particle of an elementChemical base material which cannot be chemically converted ... which cannot be chemically divided any further. The elements differ by their atomic structure. Atoms are inconceivably small. A normal drop of water contains about 6,000 quintillion (a 6 with 21 zeros) atoms. The diameter of an atom consisting of a nucleusPositively charged nucleus of an atom. Its diameter amounts ... and orbiting electrons amounts to approx. one hundred millionth of a centimetre (10-8 cm). The nucleusPositively charged nucleus of an atom. Its diameter amounts ... is made up of positively charged protons and electrically neutral neutrons. It therefore has a positive charge. Its diameter amounts to some ten trillionths of a centimetre (1 to 5 · 10-13 cm). The nucleusPositively charged nucleus of an atom. Its diameter amounts ... is therefore 100,000 times smaller than the surrounding sheath of orbiting negatively charged electrons which are as many as the protons in the nucleusPositively charged nucleus of an atom. Its diameter amounts .... Atoms therefore behave electrically neutral to the outside. See ‘nuclide’.
