Ensuring The Future – Outlook on Medical Radioisotopes and Uranium Supply Chains in Europe
Interview with Euratom Supply Agency Director General Agnieszka Kazmierczak
Medical radioisotopes are a crucial tool in beating cancer and other diseases, offering several and innovative solutions in terms of diagnostics and treatments. Despite this key role, the whole production and supply chain risk to be quite fragile in the near future.
European Nuclear Society interviewed the Euratom Supply Agency (ESA) Director General Agnieszka Kaźmierczak to learn more about the present and the future of these life-savings solutions, as well as of the nuclear fuel supply in Europe.
Which role does Europe globally have in the current medical radioisotopes production and supply?
Over 10,000 hospitals worldwide use radioisotopes in about 100 different nuclear medicine procedures, totalling almost 49 million medical procedures each year. In the EU alone, more than 1,500 nuclear medicine centres deliver about 10 million procedures to patients each year. Currently, the main source of radioisotopes is nuclear research reactors, with several other non-fission technologies such as cyclotrons and accelerators in use or under development.
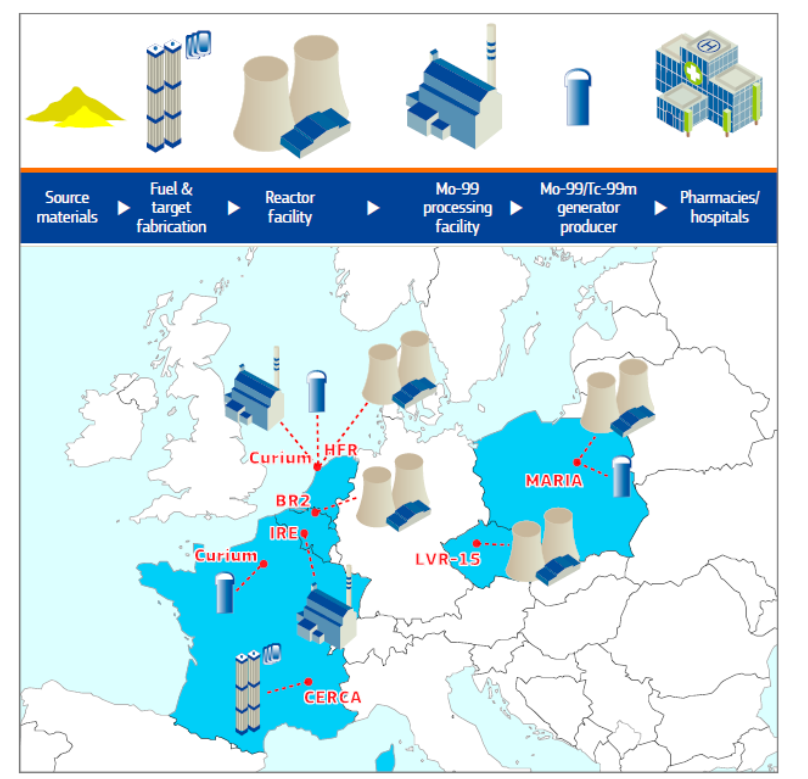
Technetium-99m (Tc-99m) is used in 80% of all nuclear medicine diagnostic procedures. The production of Tc-99m starts with irradiation of uranium targets in nuclear research reactors to produce Molybdenum-99 (Mo-99), then extraction of Mo-99 from targets in specialised processing facilities, production of Tc-99m generators and shipment to hospitals.
Any disruption to supply may have negative and sometimes severe consequences for patients.
Production of Mo-99/Tc-99m in the EU ©ESA
The EU, with a unique and complete supply chain network, plays a central role in the nuclear medicine domain, with about a 60% worldwide production share of Mo-99/Tc-99m.
How does ESA monitor the European supply and ensure the correct production and delivery procedures?
Since 2013, ESA has co-chaired, jointly with the industry association of nuclear medicine (NMEu), the European Observatory on the Supply of Medical Radioisotopes. Established in 2012, the Observatory monitors the EU supply chain of Mo-99/Tc-99m as well as discusses and supports a variety of topics related to the EU supply of widely used medical radioisotopes. The Observatory is composed of representatives of the European Commission services, international organisations and various industry stakeholders. In March 2021, the Observatory’s mission statement and terms of reference received a much-needed review. The updated documents, adopted jointly by ESA and NMEu, provide adequate governance for the challenges and the work ahead. An extended group of participants offers national governments access to expertise and information necessary for the definition of strategies and policies in this area.
Within the Observatory, the Security of Supply Working Group of the NMEu ensures effective coordination of reactor maintenance schedules to avoid and to mitigate disruptions in the supply of Mo-99/Tc-99m.
The Emergency Response Team (ERT), created within this Working Group and composed of representatives of research reactors, Mo-99 processors and Mo-99/Tc-99m generator manufacturers, monitors production and supply issues. This continuous monitoring makes it possible to identify potential shortages of Mo-99 and draw up mitigation action plans involving all stakeholders.
The Joint Communication Team (JCT), created with the Observatory, provided regular information updates received from the ERT to various stakeholder groups, including the EU administrations, OECD/NEA and IAEA.
During these last two years, how has the European supply chain been affected by the pandemic?
During the last two years in light of COVID-19 pandemic-related issues, the Observatory, in close cooperation with NMEu, monitored the uninterrupted supply of Mo-99/Tc-99m. NMEu’s ERT support was instrumental in dealing with supply disruption issues. The JCT, created with the Observatory, provided regular information updates received from the ERT to various stakeholder groups, including the Council Working Party on Atomic Questions and the Health Security Committee, OECD/NEA and IAEA.
In addition to the Observatory’s work, the Euratom Supply Agency liaised on the COVID-19 response actions with the relevant European Commission services (e.g., Directorate-General for Energy, Directorate-General for Health and Food Safety, Joint Research Centre and Directorate-General for Research and Innovation). At the dedicated meetings of the Commission’s Directorate-General for Migration and Home Affairs – COVID-19/Corona Information group – ESA debriefed EU Member States on the NMEu stakeholders’ concerns about the effect the pandemic would have on the transport of medical radioisotopes due to lockdown, extended border controls and the curtailment or elimination of many flights.
Beyond the pandemic, what do you think will be the main challenges that could impact the European medical radioisotopes supply chain in the next future? And how could Europe face the above-mentioned challenges to secure a reliable supply of radioisotopes?
Two aspects are the most important – the availability of nuclear raw materials for medical radioisotope production (HEU – high enriched uranium and HALEU – high-assay low enriched uranium) and the guarantee of existence of the irradiation infrastructure.
ESA continues to scrutinise security of supply of HEU and HALEU, which are required to feed the production of medical radioisotopes and to fuel research reactors. These strategic materials are currently not produced in the Community and must be imported from the US or the Russian Federation. We were entrusted this task under Commission’s SAMIRA initiative – Strategic Agenda for Medical Ionising Radiation Applications.
In cooperation with the Member States concerned, ESA continues to facilitate the supply of HEU to users who still need it until their conversion to HALEU, in line with international nuclear security and non-proliferation commitments. In 2021, the Agency renewed for the next five years the Memorandum of Understanding with the US Department of Energy-National Nuclear Security Administration (DoE-NNSA) for the exchange of HEU needed to supply European research reactors and medical radioisotope production facilities.
In May 2022, the working group on HALEU under the auspices of our Advisory Committee presented the finalised report on options to ensure the future supply of HALEU for EU users. One of these options is building a European capacity for producing LEU metal responding to EU needs for the research reactors’ fuel and medical radioisotopes production. The report also provides an updated view of HALEU needs, including potential EU and global demand.
As far as the production infrastructure is concerned, already in its 2014 report the Observatory warned that the existing network of reactors is fundamental to ensure the supply of medical radioisotopes to the market in the foreseeable future. The current capacity should be maintained, encouraging the necessary investments to both refurbish the current fleet if appropriate, or to replace the capacity that will be lost as the operating reactors reach the end of their lifespan.
It was confirmed in the study launched by DG ENER and published in 2019 ‘European study on medical, industrial and research applications of nuclear and radiation technology’ and further re-confirmed in 2021 study report, ‘Co-ordinated approach to the development and supply of radionuclides in the EU’, in which we read:
“If reduction of EU reliance on foreign supply is targeted, new investments are necessary in both domains, cyclotrons/accelerators and fission/activation installations, as the capability of existing installations to fulfil EU needs will deteriorate seriously”.
Indeed, current cyclotrons fleet will be unable to supply emerging PET isotopes. From 2035 onward, according to the life extension possibilities of BR-2, HFR, Maria and LVR-15, reactor’s production capacities will decline. From 2040, only Jules Horowitz Reactor (JHR, France) and Forschungsreaktor München II (FRM-II, Germany) will remain online if no new large installations are built. Their capacities are unable to cover fully EU needs, not only for Mo-99, but overall for essential therapeutic β-emitters nuclides such as Non Carrier Added Lu-177, Iodine-131 (I-131), etc.
The JHR Material Test Reactor is currently under construction at the CEA Cadarache centre. @JHRreactor
Not only radioisotopes. ESA’s mission is also to maintain regular and equitable supply of nuclear materials for all users in the European Atomic Energy Community, and the outbreak of the war in Ukraine once more highlighted the challenge of supply security. Is the supply for the EU nuclear fleet secured for next decades?
Russia’s invasion of Ukraine has massively disrupted the global supply system for all sources of energy. It has inter alia ruined the long-standing trust the EU held in a major nuclear energy partner.
In May 2022, the European Commission highlighted the importance of a coordinated action to reduce dependence on Russian nuclear materials and fuel cycle services. The REPower EU Plan states
‘Diversification options are also important for Member States currently dependent on Russia for nuclear fuel for their reactors serving either power generation or non-power uses. This requires working within the EU and with international partners to secure alternative sources of uranium and boosting the conversion, enrichment and fuel fabrication capacities available in Europe or in EU’s global partners.’
In terms of dependence on Russia, the critical short-term risk exists in fuel fabrication for one market segment – VVER reactors, mostly concentrated in a single region (Hungary, Slovakia, Bulgaria, Czechia and Finland). In addition, due to the continuing military aggression impeding routes through Ukraine, the impact of the European restrictive measures in response, as well as carriers’ reluctance to cooperate with Russians, planned deliveries of Russian nuclear material and fuel may be hindered by logistical problems.
In the medium and long term, the increased risk relates to conversion and enrichment capacity and encompasses all EU as well as other open-market countries. The outlook for the uranium mining does not seem to face undue high risk but depends on many factors and requires monitoring for early signs of instability.
As far as research reactors and radioisotope production, Russia-supplied research reactors material and fuel stocks may last at least two to three years. Some research reactors are dependent on Russian fuel supply. However a Euratom-financed research project is at an advanced stage of EU alternative fuel design testing.
Several nuclear power plants in Eastern Europe are equipped with VVER-type Russian reactors and are fully dependent on Russia’s nuclear fuel supply. Are there any plans to diversify the uranium supply and to lighten EU dependence on foreign suppliers? What other potential sources are available on the horizon?
As far as fuel fabrication is concerned, the utilities operating Russian-designed reactors are expediting efforts to make the alternative fuel available as fast as possible. The European Commission and the Euratom Supply Agency are working closely with utilities and alternative suppliers.
In April 2022, ČEZ (CZ) awarded a multiannual fuel contract for its VVER-1000 units to Framatome and Westinghouse. Framatome is planning to fabricate the fuel for VVER-1000 on Russian license in Lingen (Germany). Westinghouse has a fuel fabrication plant in Sweden and has developed its own design for both VVER 1000 and VVER 440.
The licensing process for Westinghouse fuel for VVER1000 is quite advanced in Czechia but also in Bulgaria.
As far as VVER440 is concerned, utilities from the various countries concerned are expediting the diversification process and concentrating efforts to obtain the licensing of the alternative design by Westinghouse, in compliance with the applicable national safety provisions.
As said, research reactors that are dependent on Russian fuel supply should expedite the emergence of an alternative design. The advanced stage of the research project for EU alternative fuel design testing is a step in the right direction.
ESA recently joined ENS as a new Member. What do you think are the main strong points and the mutual benefits of this new cooperation?
ESA, which has been operating since June 1960, celebrated in May of this year (with a delay due to the pandemic) its 60th anniversary, with the European Commissioner Kadri Simson for Energy delivering the keynote address at the event. Commissioner Simson stated
“for the medium and long term, we have learnt, together with the Agency, that risk preparedness, based on sound risk assessment and including diversification of supply sources, is the key means to achieve security of supply. We can see that decisions taken by any user or producer affect the whole single nuclear market. It is therefore of utmost importance to work closely together in the spirit of solidarity”.
In the interest of security of supply of nuclear materials and services, for all EU users and all civilian uses, including in the medium and long term, the Agency is entrusted with strong prerogatives, under the Euratom Treaty, it operates its Nuclear Market Observatory and publishes authoritative information and analyses. It has managed to establish itself as a considerable market player, as well as a respected and trusted interlocutor of the nuclear sector stakeholders.
We are pleased to bring these assets to ENS. On the other hand, through its ENS membership, ESA expects to widen its links with the nuclear world – to reach further professionals from academy and research, industry and authorities, to share ideas and enthusiasm with them, to deliver messages and to learn. In one phrase, for ESA, it will be both about listening and making its voice heard.